SALINAS — Four days ago, Javier Lopez couldn’t walk half a block before the pain in his right leg would stop him from taking another step.
Today, the 74-year-old smiled as he walked alongside the doctor who rejuvenated his mind and body by performing an innovative stent procedure that got the blood flowing again in Javier’s legs.
“I feel great,” said Lopez, who was a patient at Salinas Valley Health. “I knew this treatment was my best chance at having a normal life again. My disease didn’t just affect me, it impacted my whole family. I’m very thankful for Dr. Joye.”
Not only is Dr. Jim Joye at Salinas Valley Health one of the first two physicians in the nation to perform the new FDA approved procedure, Joye is also the man who 20 years ago came up with the idea for the medical breakthrough. He dreamed of an innovative approach to treat complex peripheral arterial disease (PAD) with a non-invasive technique and specialized stent to bypass a blocked femoral artery and restore blood flow to the leg.
“It feels extraordinary,” said Joye, an interventional cardiologist. “It’s been my life’s work and it took a lot of smart people to make this happen. I get emotional when I think of all of the patients this will help live longer and better lives.”
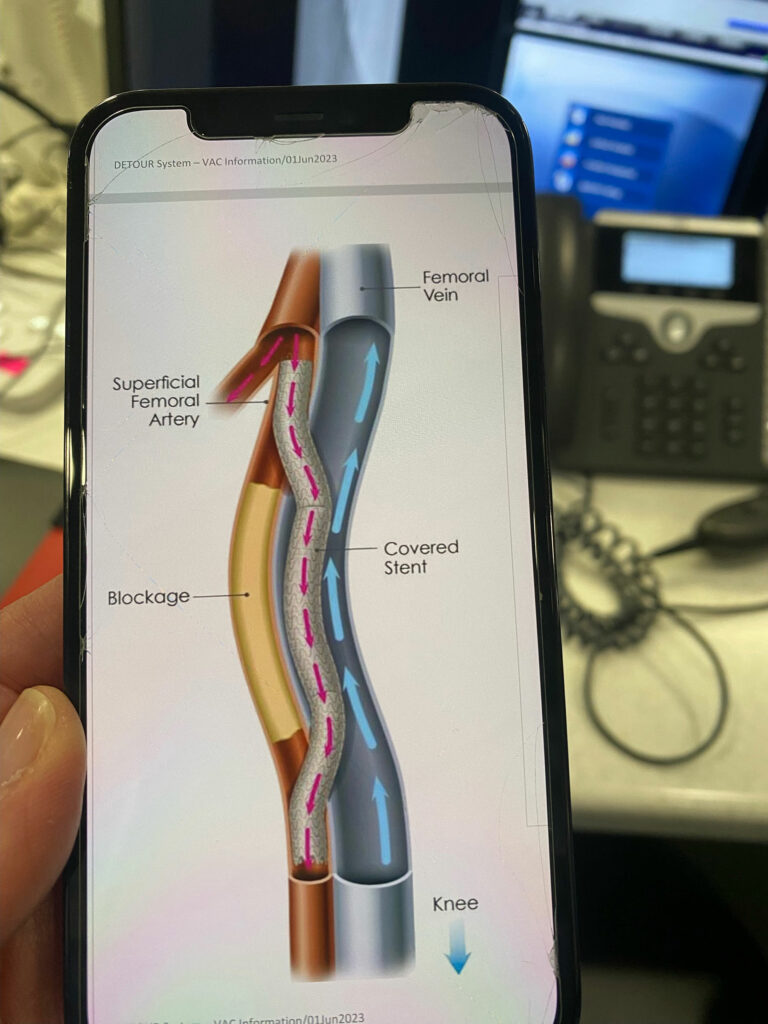
The revolutionary technique is the first-ever fully percutaneous transmural arterial bypass (PTAB) therapy using the Detour System and is an alternative to more invasive bypass surgery. Instead of days in the hospital and 6-12 weeks of recovery, patients have an overnight stay in the hospital or, like Lopez, go home the same day as the procedure and see an immediate improvement in blood flow and pain relief.
The Detour System has undergone years of global clinical trials and following FDA approval in June for commercial use in the United States, Cleveland Clinic and Salinas Valley Health were selected to launch the nationwide rollout.
“Our healthcare system has always been on the forefront of innovation and technical advancements,” said Pete Delgado, president/CEO of Salinas Valley Health. “In this case, it is especially rewarding and inspiring to see Dr. Joye’s vision come to fruition in such a profound and impactful way. Our organization is honored to be part of this historic milestone in medicine.”
The National Institutes of Health estimates 8-12 million Americans suffer from PAD or peripheral artery disease. People over 50, those with diabetes and those with a history of tobacco use are especially at risk. People with persistent and complex PAD face an increased risk of ulcers and gangrene, which can lead to limb or foot amputation.
For more information, visit salinasvalleyhealth.com/detour.